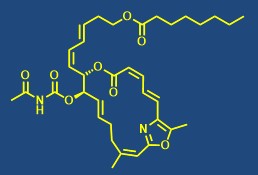
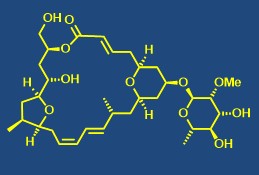
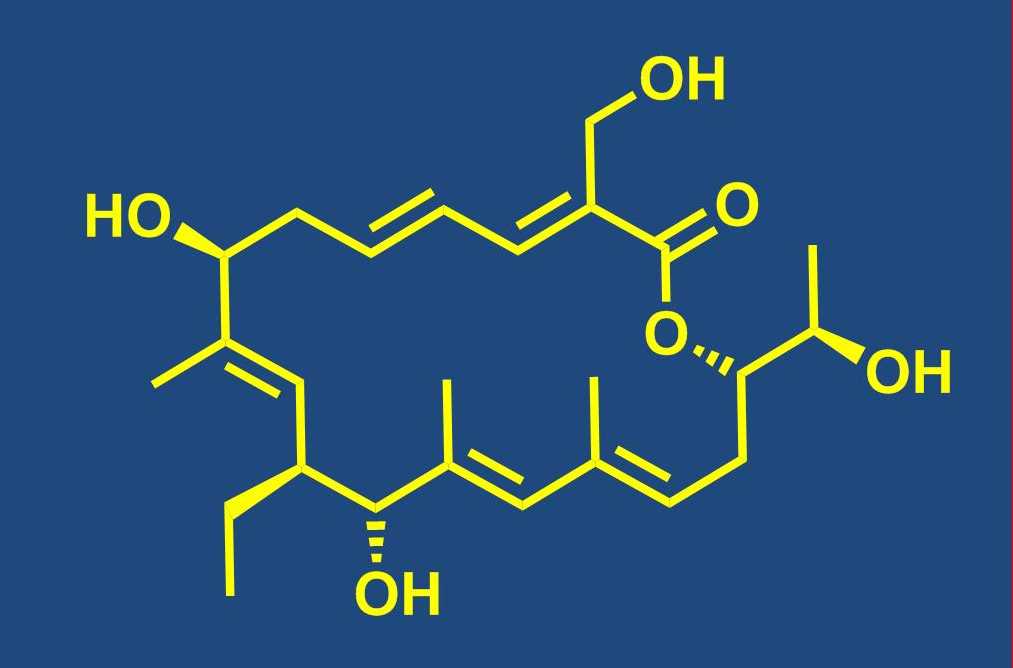
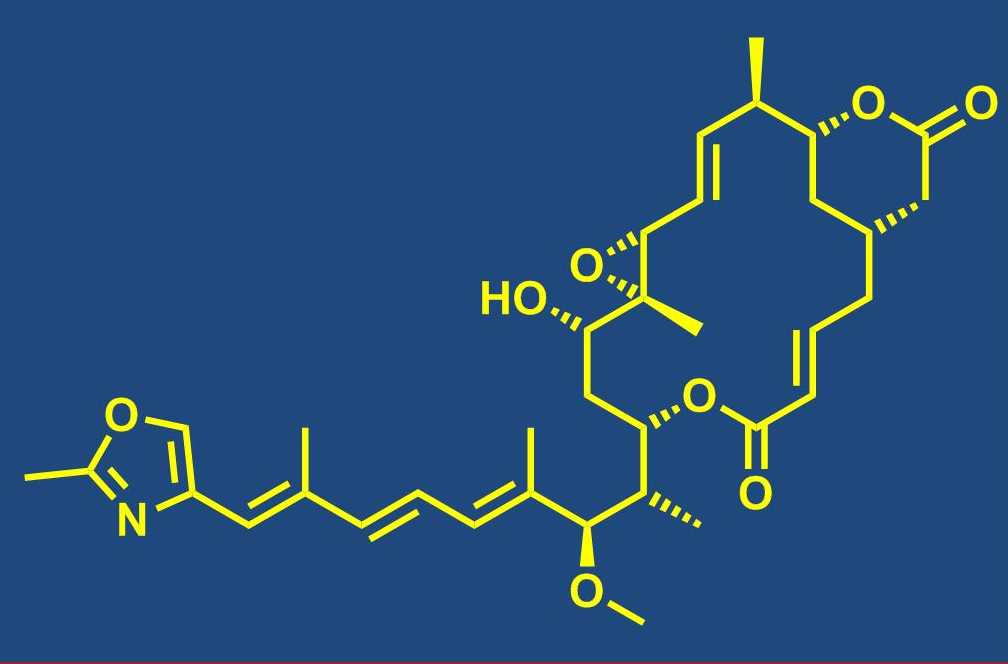
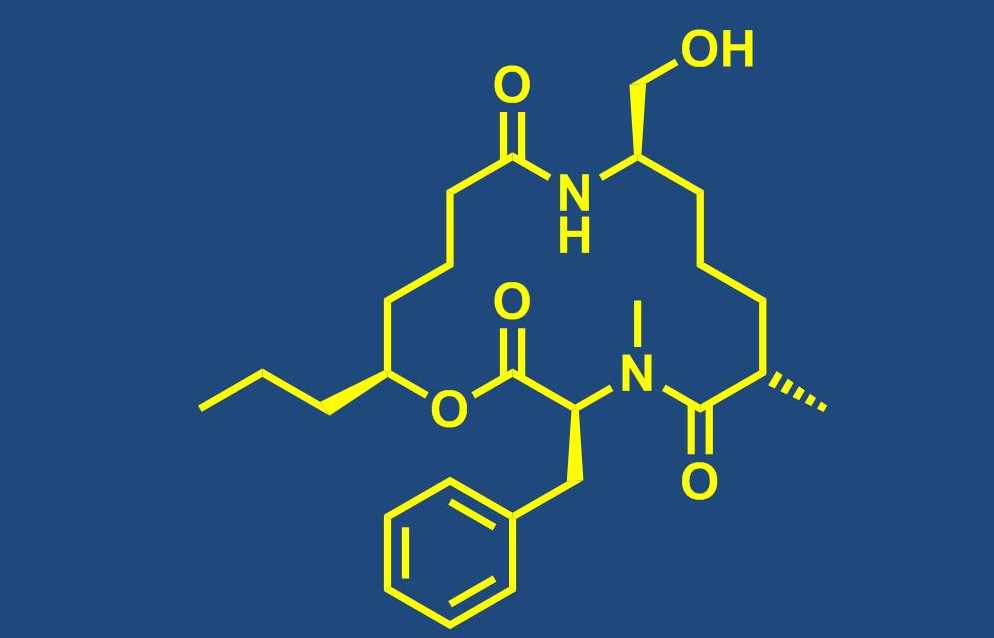
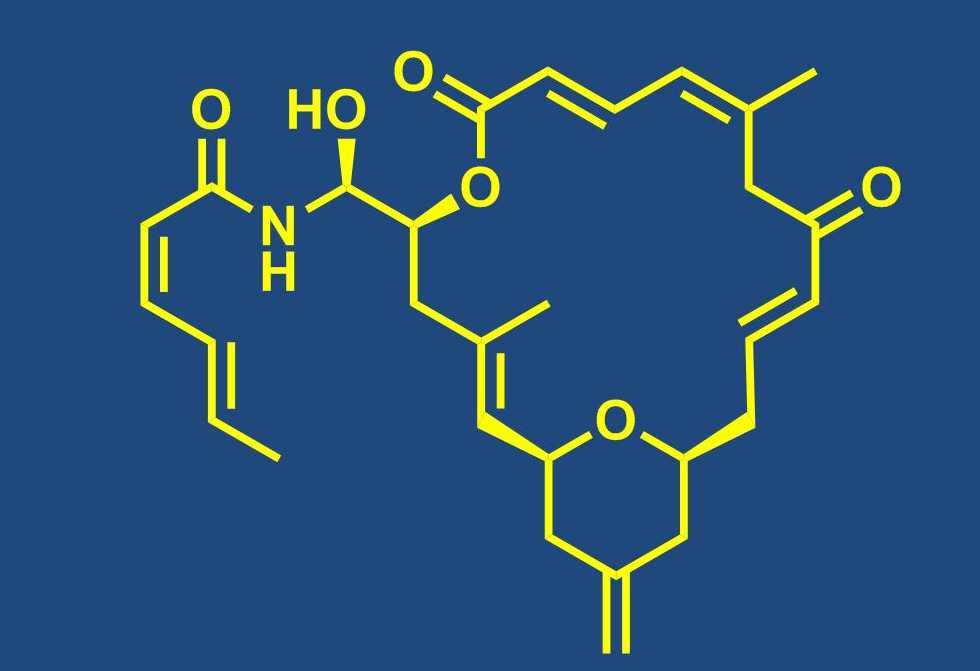
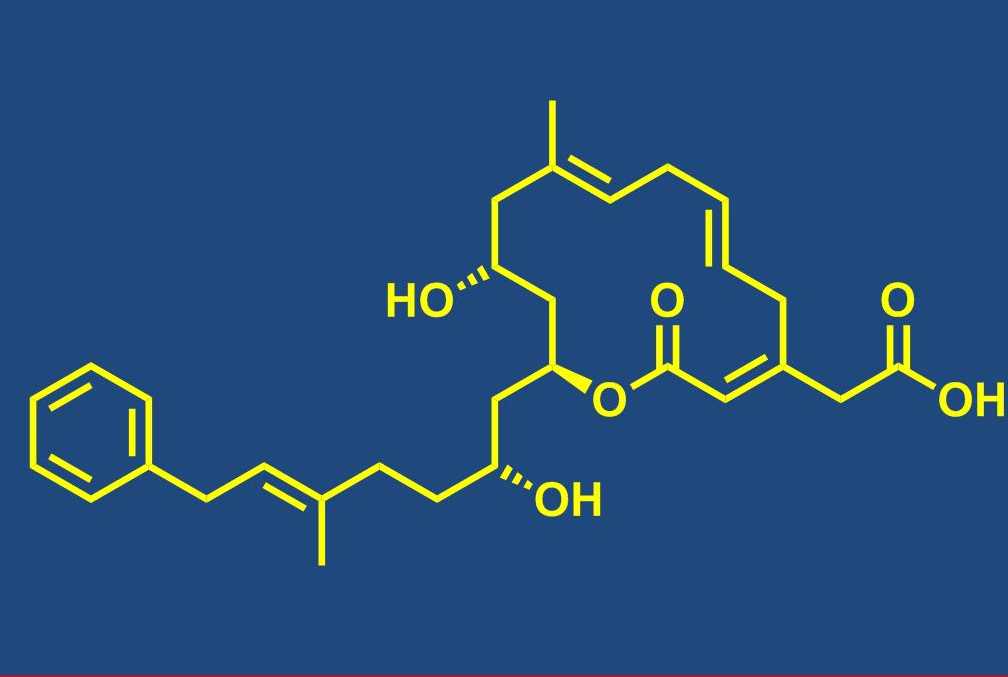
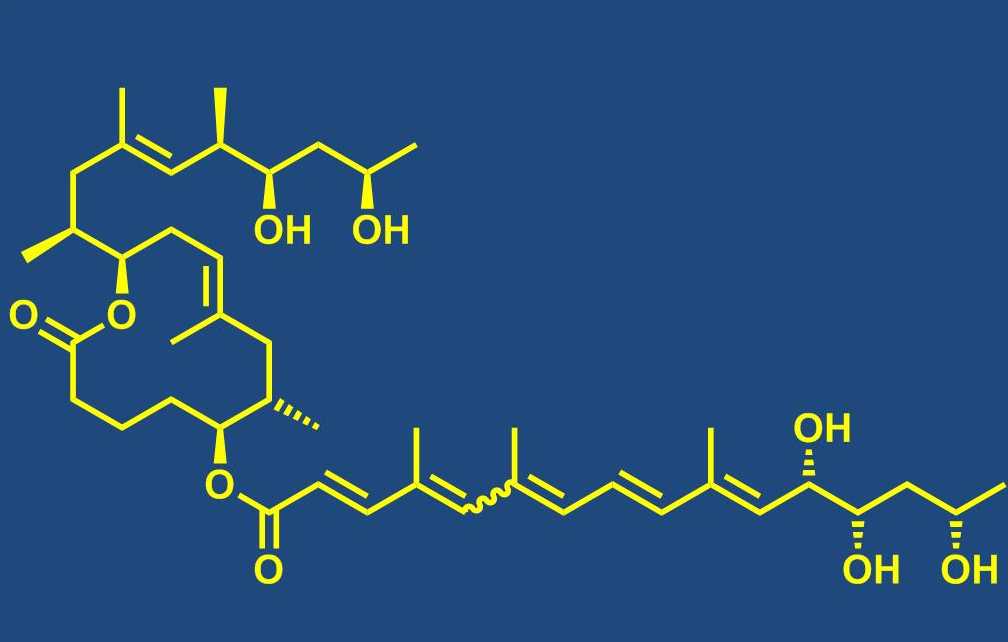
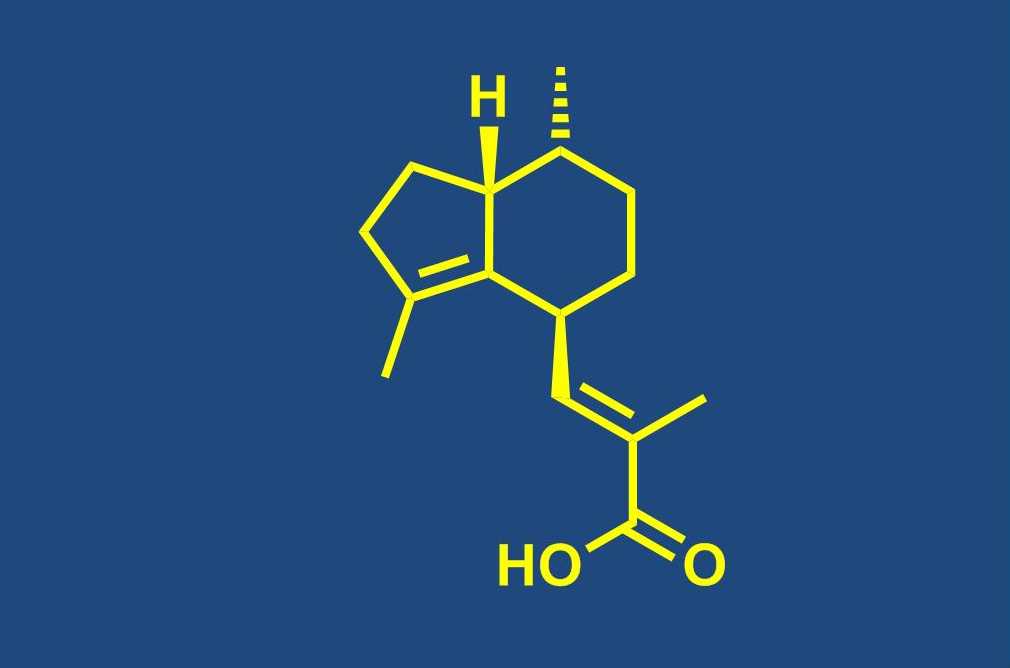
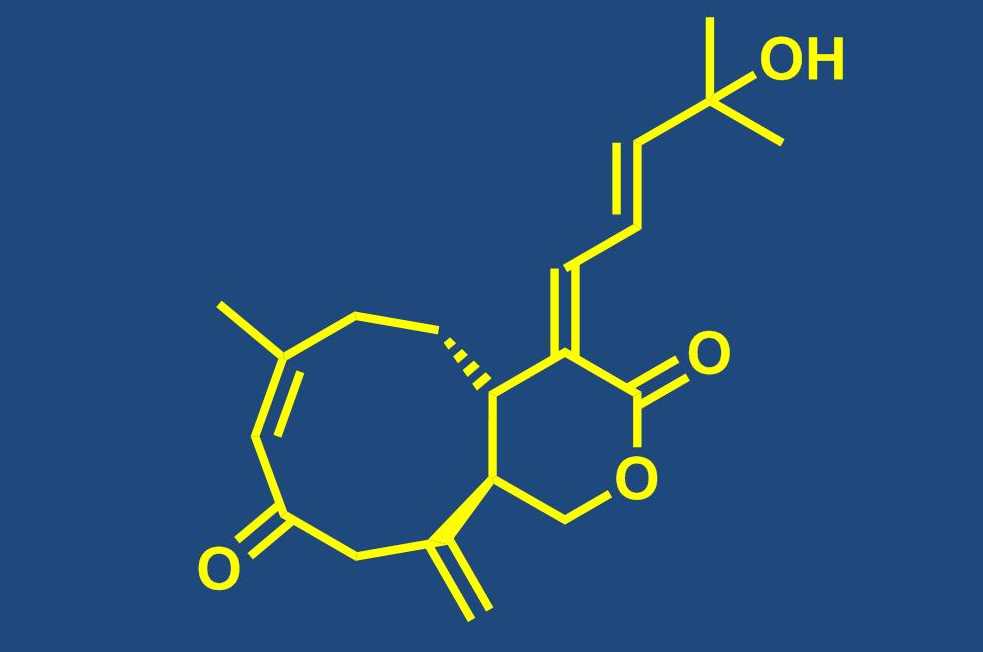
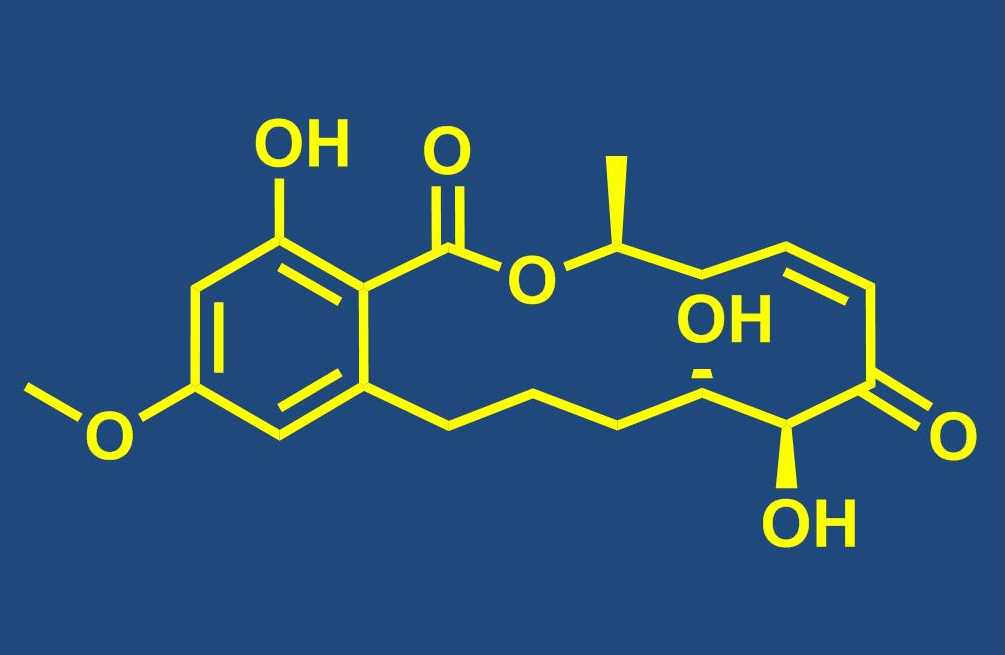
Total synthesis of bioactive natural products
Our group is engaged in the total synthesis of bioactive NPs from a variety of biological sources, including NPs that are produced by terrestrial plants, fungi or bacteria as well as organisms from the marine environment. Total synthesis has an important role to play in the continuing exploitation of NPs as leads for drug discovery, as it often represents the most efficient means (even for complex structures) to provide sufficient quantities of material for meaningful biological profiling. This is particularly true, but not limited to NPs that are obtained from marine macroorganisms. In addition, in individual cases total synthesis is still required to ascertain the full stereostructure of a new natural product (or revise a published structure). Fully synthetic NPs or NP analogs have also entered clinical trials and the approval and introduction into clinical use of the fully synthetic halichondrin B analog eribulin constitutes the preliminary culmination of these recent developments. Beyond the basic issue of substance supply, the chemistry developed in the course of a total synthesis provides the basis for analog synthesis and structure-activity-relationship (SAR) studies.
Selected reading:
Nicolaou, K. C., Rigol, S. Total Synthesis in Search of Potent Antibody-Drug Conjugate Payloads. From the Fundamentals to the Translational. Acc. Chem. Res. 2019, 52, 127-139.
F. Liu, A. G. Myers. Development of a platform for the discovery and practical synthesis of new tetracycline antibiotics. Curr. Opin. Chem. Biol. 2016, 32, 48-57.
Yu, M. J., Zheng, W., Seletsky, B. M. From micrograms to grams. Scale-up synthesis of eribulin mesylate. Nat. Prod. Rep. 2013, 30, 1158-1164.
Klar, U., Platzek, J. Asymmetric total synthesis of the epothilone sagopilone - From research to development. Synlett 2012, 23, 1291-1299.